
It does not replace medical advice from your health care provider. This is a phase II randomized double-blind active-controlled study to evaluate the safety immunogenicity and effect on infant immune responses of a single dose of Tetanus diphtheria acellular pertussis vaccine Tdap in pregnant women in Mali.
The Tdap vaccine is given during your pregnancy because it can help protect your baby against pertussis after birth.
Tdap pregnancy category. Tdap during pregnancy provides the best protection for mother and infant. Recommend and administer or refer your patients to receive Tdap during every pregnancy. Optimal timing is between 27 and 36 weeks gestation preferably during the earlier part of this period to maximize the maternal antibody response and passive antibody transfer to the infant.
Pregnancy Category C. Tdap Adacel Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans but potential benefits may warrant use of the drug in pregnant women despite potential risks OR Animal reproduction studies have not been conducted and there are no adequate and well-controlled studies in humans. Diphtheria toxoid pertussis acellular tetanus toxoid Pregnancy Warnings.
This drug should be used during pregnancy only if clearly needed. AU TGA pregnancy category. B2 US FDA pregnancy category.
Not assigned Comments-There is no data on use in pregnant women to know this drugs risks including the risk of fetal harm or reproductive effects. In many countries the use of acellular pertussis adult vaccine in combination with tetanus and diphtheria toxoids Tdap is recommended for women during pregnancy to protect newborns in the first months of life when they are too young to be vaccinated. Objectives Actively recruit and intensively follow pregnant women receiving a dose of acellular pertussis vaccine for 4 weeks after vaccination.
Design and settings A prospective observational study conducted in 2 New Zealand regions. Participants Women in their 28th38th week of pregnancy recruited from primary care and antenatal clinics at the time of Tdap administration. Maternal Tdap vaccination was captured from the EMR and defined as receipt of the vaccine during any point during the pregnancy and an ASD was defined by a clinical diangosis any time after turning one year of age as recorded in the EMR between January 1 2012 and June 30 2017.
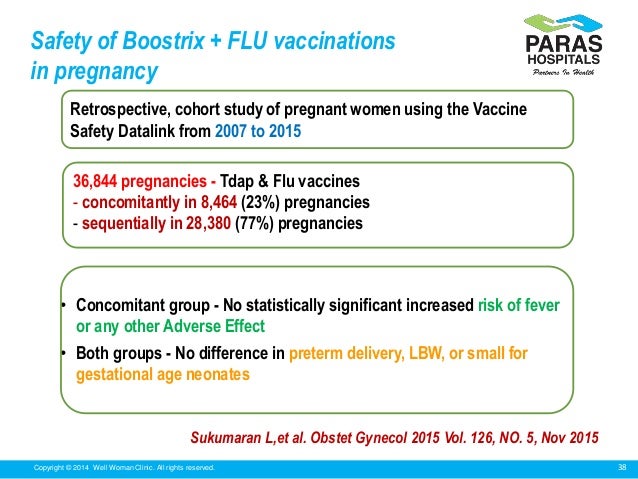
However few published studies have evaluated the safety of Tdap vaccine in pregnant women. 7-11 In these studies Tdap vaccination during pregnancy has not been associated with an increased risk of spontaneous abortion stillbirth preterm delivery low birth weight neonatal complications or congenital anomalies compared with unvaccinated pregnant controls. Video Abstract BACKGROUND.
Increasing vaccination of pregnant women makes it important to assess safety events potentially linked to prenatal vaccination. This study investigates the association between prenatal tetanus diphtheria acellular pertussis Tdap vaccination and autism spectrum disorder ASD risk in offspring. This is a retrospective cohort study of mother.
This is a phase II randomized double-blind active-controlled study to evaluate the safety immunogenicity and effect on infant immune responses of a single dose of Tetanus diphtheria acellular pertussis vaccine Tdap in pregnant women in Mali. 200 healthy pregnant women ages 18 through 39 years inclusive who meet all eligibility criteria will be randomly allocated in a 21 ratio to. When you get the Tdap vaccine during pregnancy it helps protects your baby from whooping cough or pertussis through passive immunity immunity thats passed from mother to child.
This matters because if a baby gets pertussis as a newborn it can be fatal and babies cant be protected with their own vaccination until they are 2 months old. Newborns are the most likely to be hospitalized. The study objective was to evaluate patient reaction following receipt of Tdap vaccination during pregnancy.
This was a prospective observational study conducted from May 2014 through March 2016. The study design involved solicited patient reaction within 1-7days after the administration of the Tdap vaccine. Data collected included pain or soreness swelling andor redness at the.
The Tdap vaccine is given during your pregnancy because it can help protect your baby against pertussis after birth. Once you receive the vaccine your body produces high levels of pertussis antibodies which you pass onto your baby protecting him from contracting whooping cough from the point he is born until he is able to get his shots. In the past Tdap was not recommended during pregnancy because pertussis was uncommon in adults but this is no longer the case.
It is now recommended that pregnant women get the vaccine during the third trimester of pregnancy between weeks 27-36. However it can be given anytime during pregnancy. Receiving the shot in the third trimester is suggested so the baby can get.
To ensure better protection for your baby optimal timing for Tdap administration is between 27 and 36 weeks of gestation although Tdap may be given at any time during pregnancy. Pertussis vaccination during pregnancy is now officially recommended in several countries including the United States United Kingdom Canada New Zealand Belgium Brazil Argentina Mexico and Israel to protect infants. Tdap Vaccine in Pregnancy - 2 - Disclaimer.
This document contains information andor instructional materials developed by Michigan Medicine for the typical patient with your condition. It may include links to online content that was not created by Michigan Medicine and for which Michigan Medicine does not assume responsibility. It does not replace medical advice from your health care provider.
Pregnancy registry by calling 1-888-452-9622. 81 See 17 for PATIENT COUNSELING INFORMATION. CONTENTS 1 INDICATIONS AND USAGE 2 DOSAGE AND ADMINISTRATION.
Tdap after pregnancy was a time-varying covariate that indicated maternal Tdap vaccination from the day of birth onwards. The 3 main indicator variables of maternal Tdap vaccination status in the analyses before pregnancy during pregnancy at least 8 days before birth and after pregnancy are not exclusive. It was possible to receive Tdap during 1 of these periods.