
Animal model studies show an association between maternal immune activation during pregnancy and behavioral and brain abnormality in offspring similar to those observed in children with ASD. Vaccination during pregnancy is estimated to reduce the risk of influenza in babies aged less than 6 months by about half.
Vaccination has been shown to reduce the risk of flu-associated acute respiratory infection in pregnant women by up to one-half.
Influenza vaccine during pregnancy. Getting an influenza flu vaccine is the first and most important step in protecting against flu. Pregnant women should get a flu shot and not the nasal spray flu vaccine. Flu shots given during pregnancy help protect both the mother and her baby from flu.
Vaccination has been shown to reduce the risk of flu-associated acute respiratory infection in pregnant women by up to one-half. Influenza Vaccination During Pregnancy Recommendations. The Centers for Disease Control and Preventions CDC Advisory Committee on Immunization Practices and.
Published data continue to demonstrate the need for influenza vaccination during pregnancy. After vaccination pregnant women have similar protective titers of anti-influenza antibodies as non-pregnant women demonstrating that pregnancy does not alter the trivalent inactivated influenza vaccine immune response. Studies from the United States Europe and resource-constrained regions demonstrate that maternal vaccination is associated with increased anti-influenza antibody.
This systematic review aimed to synthesize the best available evidence on the safety of influenza vaccination during pregnancy on fetal development. A search of the literature was undertaken from the inception of each database up to March 2014. Both observational and clinical trials were considered.
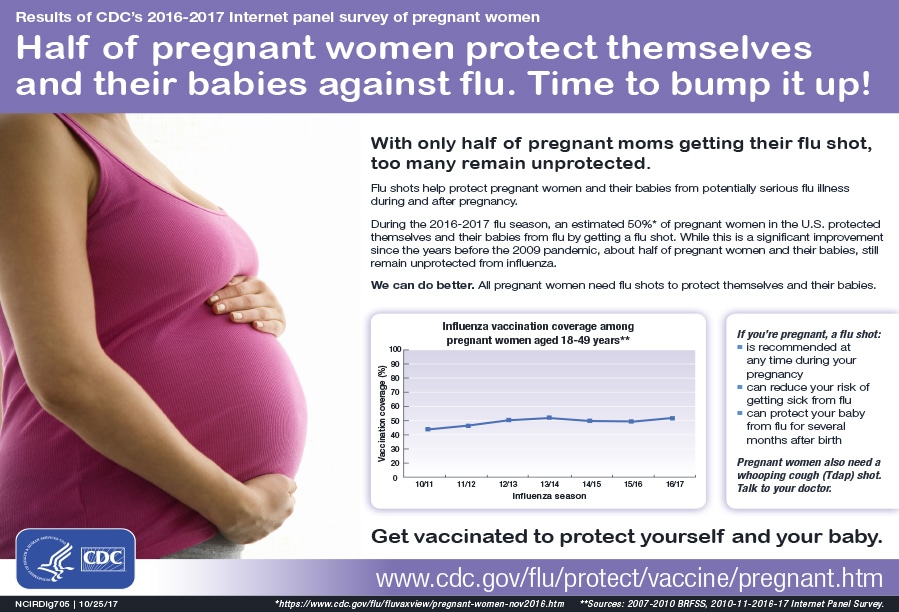
Get a flu shot anytime during each pregnancy. CDC recommends getting a flu vaccine by the end of October despite flu seasons varying in their timing from season to season. This timing helps protect a pregnant woman before flu activity begins to increase.
To improve uptake of influenza vaccine in pregnancy it is important to understand the factors that predict prenatal vaccination. The aim of this study was to test the capability of the theory of p. Influenza vaccination can be administered at any time during pregnancy before and during the influenza season.
Women who are or will be pregnant during influenza season should receive IIV. Influenza LAIV Live attenuated influenza vaccine LAIV is not recommended for use during pregnancy. 5 Top of Page.
Measles Mumps Rubella MMR. There is level 2b evidence that maternal health and pregnancy outcome can be severely affected by influenza virus infection. Antiviral treatment may diminish these effects and vaccination protects pregnant women and neonates from infection level of evidence 2b and 1b respectively.
Vaccination during pregnancy is estimated to reduce the risk of influenza in babies aged less than 6 months by about half. Vaccination timing Influenza vaccine is recommended as a single dose at any time as early as practicable during each pregnancy. It is best given prior to the onset of the influenza season.
Influenza vaccination of women during pregnancy Committee reports. 14 July 2006 - Safety of pandemic influenza vaccines from meeting of 6-7 June 2006 16 January 2004 - Influenza vaccination of women during pregnancy from meeting 3-4 December 2003 Related links. WHO position paper on influenza vaccines - WER 19 August 2005 pdf 214kb.
In addition preliminary data from a few studies of influenza vaccine in pregnant women have confirmed not only the benefit of providing protection in this vulnerable population but positive effects in their infants including the reduction of low birth weights and a significant decrease in influenza-related pneumonia in young children. It was pointed out that manufacturers as well as national regulatory authorities tend to caution against routine use of influenza vaccine during pregnancy. Despite the paucity of data elated to the use of influenza vaccines during the first trimester of pregnancy other inactivated vaccines eg.
Tetanus have proved safe in this context. No evidence indicates that killed influenza vaccine is teratogenic even if given during the first trimester. Since 1996 Health Canadas Centre for Disease Control and Prevention has recommended that pregnant women in their second and third trimesters be vaccinated.
This should not be interpreted as evidence that the vaccine is teratogenic in the first trimester because such evidence does not exist. The best way to protect your newborn baby against influenza is to get vaccinated during pregnancy. The influenza vaccine is free for pregnant women as part of the National Immunisation Program NIP.
The influenza vaccine is recommended during every pregnancy and at any stage of your pregnancy. If you are pregnant you should get the flu vaccine because you are at increased risk of severe complications from flu. The vaccine protects you during pregnancy.
You can get the flu vaccine at any stage of pregnancy. You should get it as early as possible in your pregnancy. Influenza vaccine is free for pregnant women through the National Immunisation Program.
When is the best time to have the vaccine during pregnancy. The influenza vaccine can. Animal model studies show an association between maternal immune activation during pregnancy and behavioral and brain abnormality in offspring similar to those observed in children with ASD.
17-19 Pregnant women are encouraged to get vaccinated against influenza because they face an increased risk of complications from the infection. 20 Studies show that influenza vaccination during pregnancy. Vaccination against influenza flu during pregnancy is recommended for all women especially during flu season November to April.
This is because flu is more likely to cause severe illness in pregnant women than in women who are not pregnant.